The first molecular bond in the universe has finally been detected in space
After decades of searching, scientists have finally detected in space the first molecular bond that would have formed in the early Universe.
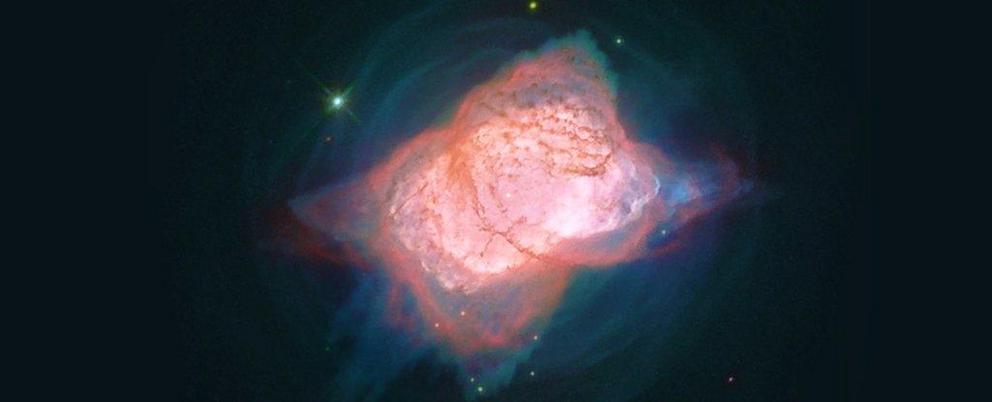
The unambiguous discovery of the helium hydride ion HeH+ in the planetary nebula NGC 7027 brings to a close an epic hunt to locate the elusive molecule in outer space, and cements theoretical predictions of the chemistry that essentially makes the Universe as we know it possible.
"The lack of evidence of the very existence of helium hydride in the local Universe has called into question our understanding of the chemistry in the early Universe," astronomer Rolf Güsten told ScienceAlert.
"The detection reported now resolves such doubts."
Once the early Universe cooled down following the Big Bang almost 14 billion years ago, theory suggests that the ions of light elements began to recombine with one another.
At a temperature somewhere below 4,000 Kelvin, the early Universe bore witness to what researchers say was the dawn of chemistry, and the whole process – according to science – depended on one pivotal step.
"In this metal-free and low-density environment, neutral helium atoms formed the Universe's first molecular bond in the helium hydride ion HeH+ through radiative association with protons," Güsten and fellow researchers explain in a new paper.
On an understandably smaller scale, scientists replicated the basic chemistry in the lab almost as far back as a century ago – but one considerable hurdle remained.
That hurdle was that helium hydride – this most elementary of elementary compounds – was never seen in the wild. By wild, we mean space, and by space, we mean planetary nebulae.
Planetary nebulae are glowing, expanding clouds of ionised gas that are expelled in the last stages of a star's life – and they're one of the closest astronomical analogues we have for post-Big Bang chemistry, at least as far as HeH+ is concerned.
Scientists predicted HeH+ might form in planetary nebulae back in the 1970s, but up until now we'd still never been able to detect it.
According to the researchers, that's because Earth's atmosphere is essentially a brick wall for ground-based spectrometers trying to perceive the molecule at the specific infra-red wavelength where it would be viewable.
In addition, previous technological limitations in comparative low-resolution spectrometry made any observations of HeH+ ambiguous at best.
Güsten's team was able to overcome these two barriers in unison, thanks to the capabilities of the German Receiver for Astronomy at Terahertz Frequencies (GREAT) when flown aboard NASA's Stratospheric Observatory for Infrared Astronomy (SOFIA) aircraft.
According to Güsten, GREAT is the only instrument worldwide that can perform these kinds of observations, and it would only ever be capable of seeing helium hydride in space if it were airborne first.
"One cannot perform this search from ground-based observatories because at [the] 149 μm wavelength, Earth's atmosphere is totally opaque," Güsten says.
"So you need to go into space or operate your instrument from a high-flying platform like SOFIA, cruising above the absorbing lower atmosphere."
And that's what they did.
Over three flights in May 2016, the team used their high-resolution spectrometer to observe the planetary nebula NGC 7027, and the readings gave the scientists exactly what they were looking for: the first unambiguous signal of the first ever molecule in space (after the Big Bang at least).
Güsten says, with the new NGC 7027 results in hand, we can now put constraints on the chemical reactions that control the formation and destruction of the helium hydride molecule.
"The respective rates are difficult to measure/to calculate, and in the literature have changed by factor of 10 in recent years," Güsten told ScienceAlert.
"Our observations will help to 'calibrate' these rates, and this will feed-back into the chemical 'networks' of the early Universe."
The findings are reported in Nature.