Organ-on-a-chip: 3-D self-rolled biosensor array to electrically interrogate electrogenic cells
Cell to cell communication plays an important role in coordinating the function of biological systems. Three-dimensional (3-D) spheroids (cell aggregates) allow biologists to explore cellular communication during tissue development and drug discovery since their 3-D architecture can mimic in vivo microenvironments in the lab. Cellular electrophysiology is an existing signaling technique to study electroactive cells (heart cells, neurons). However, the technique does not yet provide direct and simultaneous investigations of tissues at multiple sites in 3-D. In a new report now published on Science Advances, Anna Kalmykov and an interdisciplinary research team in the departments of Biomedical Engineering, Mechanical and Aerospace Engineering, Chemistry, Materials Science and Engineering in the U.S. and Singapore, developed 3-D, self-rolled biosensor arrays (3-D-SR-BA). They built-in active field effective transistors or passive microelectrodes within the devices to interface with encapsulated 3-D human cardiac spheroids to investigate electrogenic cell behavior.
Using the arrays, the research team acquired continuous and stable multiplexed recordings of field potentials with high sensitivity and spatiotemporal resolution alongside simultaneous calcium imaging. The approach allowed the scientists to conduct electrophysiological investigations to monitor complex signal transduction within 3-D cell aggregates on an organ-on-an-electronic-chip platform (organ-on-e-chip). The work will pave the way to investigate live tissue maturation and cell differentiation in the lab, while supporting electroactive cell-based drug development strategies to treat diseases such as arrhythmias.
Multicellular life is based on cell to cell communication, which forms the foundation of higher-order functions in a variety of tissues and organs. Cells are intimately connected to each other in their native 3-D microenvironment and to the surrounding matrix to form a complex and dynamic system. Bioscientists have typically cultured cells on 2-D surfaces (cell culture plates) for decades for in vitro studies. However, both 2-D and 3-D culturing techniques quantitatively and qualitatively differ in many key characteristics of cellular behavior.
Geometric conformation of the 3-D self-rolled biosensor array (3-D-SR-BA) on release from the sacrificial layer etch. Credit: Science Advances, doi: 10.1126/sciadv.aax0729
Cellular electrophysiology is used to study cell communication across a wide range of cells, including electroactive cardiomyocytes (CMs), neurons and α/β cells in pancreatic isles, and non-electroactive cells including hepatocytes and immune cells. 3-D organoids cultured on organ-on-chip platforms have formed a new avenue to investigate tissue development and for drug discovery strategies. Bioengineers have used such systems to study the mechanisms underlying cell-cell communication, with potential applications in tissue engineering.
In the present work, Kalmykov et al. presented a new, combined approach to engineer an organ-on-electronic-chip as a self-rolled biosensor array (3-D-SR-BA) and conducted electrophysiological measurements of spheroids to study 3-D multicellular systems. Materials scientists are currently developing intriguing and evolving bioactive advanced materials while investigating self-rolled polymeric structures with live cells to understand cell-material motion/actuation in response to light, pH, temperature and electrical or magnetic triggers.

Highly controlled 3-D-SR-BAs. (A to C) Bright-field optical microscopy images of photolithographically fabricated 3-D devices. (A) As fabricated 3-D-SR-BA before being released. (B) 3-D-SR-BA released with a single turn. (C) 3-D-SR-BA released with ~1.7 turns. Scale bars, 100 μm. (D and E) 3-D-SR-BAs with varying radii of curvature — simulation and experimental results. (D) 3-D-SR-BA with a single turn. (I) A finite element analysis (FEA) simulation result for a 3-D-SR-BA with a single turn (inner diameter of ~160 μm). Color bar represents the magnitude of the displacement of the 3-D-SR-BA upon removal of the sacrificial layer; SU-8 layers are not shown for visual purpose. (II) A 3-D confocal microscopy image of a representative 3-D-SR-BA with a single turn (n = 9). (E) 3-D-SR-BA with multiple turns. (I) FEA simulation result for a 3-D-SR-BA with ~1.7 turns (inner diameter, ~100 μm). Color bar represents the magnitude of the displacement of the 3-D-SR-BA upon removal of the sacrificial layer; SU-8 layers are not shown for visual purpose. (II) 3-D confocal microscopy image of a representative 3-D-SR-BAs with ~1.7 turns (n = 15). Scale bars, 50 μm.
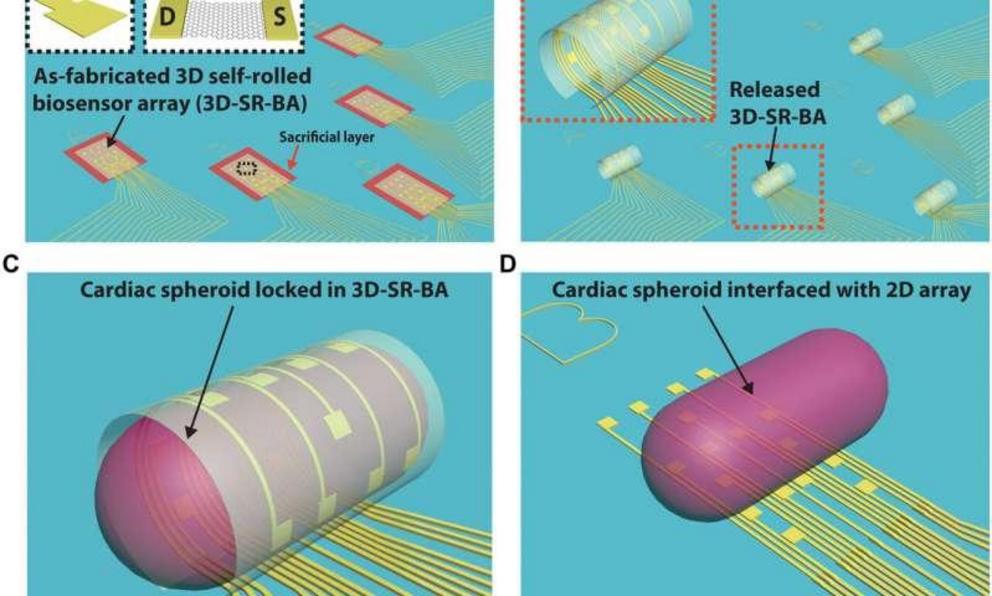
For the present strategy, the research team developed a controlled assembly of electrical biosensors in 3-D with prestressed metal/polymer support multilayer systems as the working platform. The research team engineered the self-rolling platform on a planar surface, which when released from the surface, achieved a controlled 3-D geometry. They studied the propagation of electrical signals within stem cell-derived, engineered cardiac cell assemblies using the approach. The system was ideal to understand cell-cell communication processes in 3-D spheroid systems.
The 3-D device (3-D-SR-BA) provided a new approach to record electrophysiological signals and understand signal transduction in complex cellular assemblies. For instance, the knowledge of electric information propagation in cardiac spheroids can improve the basis of signal transduction in complex cellular assemblies relative to electrical-signal based diseases such as arrhythmias. This organ-on-e-chip platform will enable tissue maturation investigations on the bioelectronic platform to test the efficacy of new drug treatment regimes in the future.

TOP: 3-D-SR-BA with functional passive biosensors (microelectrodes). (A) 3-D confocal microscopy image of 3-D-SR-BA with microelectrodes. Color bar represents the depth in micrometers. Scale bar, 50 μm. (B) Representative cyclic voltammograms (CV) acquired with 1 M KCl at 600 mV/s before (blue trace) and after (red trace) PEDOT:PSS electrodeposition (n = 10). (C) Electrochemical impedance spectroscopy (EIS) plots for the electrodes before (blue trace) and after (red trace) PEDOT: PSS electrodeposition (n = 23). BOTTOM: Effect of 3-D-SR-BA on viability of CMs in an encapsulated spheroid. (A) Live/Dead assay performed on CM spheroids: a spheroid encapsulated by 3-D-SR-BA (top), a spheroid non-encapsulated in 3-D-SR-BA (bottom), imaged at (i) 0 hours (immediately after encapsulation), (ii) 1 hour, (iii) 2 hours, and (iv) 3 hours. Green, red, and blue denote live cells, dead cells, and cell nuclei, respectively. Scale bars, 100 μm. (B) Viability analysis of spheroids encapsulated by 3-D-SR-BA (blue) and not encapsulated spheroid controls (red) that were imaged every 30 min for 3 hours. Results are reported as mean ± SD (n = 3). There was no significant difference in the % viability between the encapsulated spheroids and the non-encapsulated control spheroids.
The research team powered the 3-D sensing device using its tunable character, which they controlled via electrode arrangement and device curvature. Using the self-rolled device, they obtained 3-D measurements of tissue-scale electrophysiology that were previously inaccessible with conventional electronics on 2-D chip surfaces. The ability to measure the electrical activity of the entire 3-D construct allowed them to understand signal propagation in the construct as a whole. Kalmykov et al. engineered the device on a sacrificial layer and polymeric support with metallic electrode lines to provide source and drain interconnects to the constituent field effective transistors (FETs).
To drive shape transformation of the 3-D device, the team explored the residual mismatch stress between the constituent layers of the device, which they controlled by varying the material deposition pressure, deposition rate and thickness of the final film. To understand the self-rolling behavior of the device, they modeled the process with 3-D finite element analysis (FEA). Based on the results they optimized the device to directly monitor the electrical activity of spheroids varying from size ranges of 50 to 200 µm for improved biosensor-cell interface interactions while maintaining healthy and intact spheroids.

LEFT: Electrical recordings in 3-D of cardiac spheroids. (A) A 3-D confocal microscopy image of 3-D cardiac spheroid labeled with Ca2+ indicator dye (Fluo-4, green fluorescence) encapsulated by the 3-D-SR-BA. Scale bar, 50 μm. (B) 2-D map of the microelectrodes labeled in (A). (C) Representative field potential (FP) traces recorded from the channels labeled in (A) and (B). A.U., arbitrary units. Simultaneously recorded Ca2+ fluorescence intensity as a function of time of the ROI marked by pink box in (A). (D) Averaged FP peak (red trace) and raw data (gray traces, n = 100 peaks recorded by channel 4). RIGHT: Mapping electrical signal propagation in 3-D using 3-D-SR-BA.(A) Representative recorded single FP fast transients across 12 channels. Red and blue arrows represent the positive and negative phases of the recorded transients, respectively. (B) 2-D representation of the fast transient signal phases across all 12 channels. Resting state is presented at t = 14.9 ms, and depolarizing wave propagation is presented at t = 22.0 ms and t = 27.5 ms. (C) 3-D rendered signal propagation at t = 22.7 ms. Scale bar, 50 μm. (D) 2-D representation of the isochronal map of time latencies. Scale bar, 35 μm. White arrow in (C) and (D) represents average conduction velocity direction.
To illustrate the flexibility of the 3-D device, the research team also included graphene FETs (GFETs) as active biosensors on them. In parallel, they engineered passive biosensors such as microelectrode arrays (MEAs) to study the electrophysiology of cellular networks for precise electrical stimulation on multiple sites. As a proof-of-concept, Kalmykov et al. used MEAs on the 3-D device to investigate the electrical activities of spheroids at cellular resolution. They confirmed the electrochemical properties of the built-in microelectrodes using cyclic voltammetry (CV) and electrochemical impedance (EIS) and modified the biosensors for improved electrical recording and improved electrochemical activity. For 3-D devices with active biosensors (GFETs) the research team included the sensors due to their exceptional electrical conductivity, superior robustness, mechanical strength and ease of tunability. The team then conducted biocompatibility tests of the 3-D device using cell-material viability assays with five-day old cardiomyocyte (CM) spheroids treated with blebbistatin to inhibit spontaneous cell beating. The results showed that the 3-D device did not negatively affect the encapsulated spheroid's health or viability.
During further experiments, the research team obtained 3-D recordings from human embryonic stem cell derived cardiomyocyte spheroids, which showed spontaneous contraction by day 3 of seeding. By day 7, they transferred the electrogenic cells to the 3-D device for successful encapsulation in contact with biosensors for improved cell interface interactions to obtain electrophysiological recordings. The team found stable recordings from the same spheroid up to 3 hours after encapsulation, including beat frequency information at high temporal resolution. They obtained the electrical recordings and constructed 3-D isochronal maps of the spheroid's surface and calculated the velocity of conduction, which agreed with previous reports.
In this way, Anna Kalmykov and co-workers experimentally demonstrated the first study on multisite, simultaneous measurements of a 3-D multicellular system using 3-D-SR-BA devices. The team controlled the 3-D device geometry for sensor customized spheroid interfaces of varying sizes. The experimental setup provided information from individual cells in the 3-D spheroid with exceptional recordings. The device proposed by Kalmykov et al. introduced a new approach for organ-on-electronic-chip bioelectronics. The researchers aim to extend the electrophysiological capabilities and combine electrical measurement stimulation with biosensing for more complex setups to gain deeper insight into 3-D electrogenic tissue constructs.
More information: Anna Kalmykov et al. Organ-on-e-chip: Three-dimensional self-rolled biosensor array for electrical interrogations of human electrogenic spheroids, Science Advances (2019). DOI: 10.1126/sciadv.aax0729
Stacey A. Maskarinec et al. Quantifying cellular traction forces in three dimensions, Proceedings of the National Academy of Sciences (2009). DOI: 10.1073/pnas.0904565106
Massimo Scanziani et al. Electrophysiology in the age of light, Nature (2009). DOI: 10.1038/nature08540
Journal information: Science Advances , Nature , Proceedings of the National Academy of Sciences
Video can be accessed at source link below.