Newly discovered inorganic material has the lowest thermal conductivity yet
Artist's impression of the material's atomic structure.
We all know the world desperately needs to transition to renewable power sources – but many of us forget we also need to make our energy systems themselves more efficient.
At the moment, an estimated 70 percent of all energy we generate in the world is lost as heat – often in power plants themselves. It's a significant problem that could be at least partially solved through the improvement of thermoelectric materials, which could reduce heat loss and also capture wasted heat energy.
Now researchers led by the University of Liverpool in the UK have taken a major step towards this goal, with the discovery of a new inorganic material that has the lowest thermal conductivity ever reported.
In fact, at room temperature, the material slows down the transfer of heat almost as much as air does.
The new material has the formula Bi4O4SeCl2 (not a catchy name, we know) and its creation is a "breakthrough in the control of heat flow at the atomic scale", a press release explains.
"The material we have discovered has the lowest thermal conductivity of any inorganic solid and is nearly as poor a conductor of heat as air itself," says chemist and team leader Matt Rosseinsky from the University of Liverpool.
"The implications of this discovery are significant, both for fundamental scientific understanding and for practical applications in thermoelectric devices that harvest waste heat and as thermal barrier coatings for more efficient gas turbines."
If you consider the thermal conductivity of steel as 1, then water and a construction brick would both have thermal conductivity of 0.01. Air would be around 0.0005, and the new material is just 0.001.
What's particularly exciting is that this material was created through a clever arrangement of layers of atoms, and the team says they can use the same technique to add additional properties.
In the future, that could mean crafting materials that not only are incredibly resistant to heat, but are also superconductors of electricity – two properties that would be extremely beneficial to an energy grid.
"Beyond heat transport, this strategy could be applied to other important fundamental physical properties such as magnetism and superconductivity, leading to lower energy computing and more efficient transport of electricity," explains physicist Jon Alaria.
Inorganic materials are those that don't contain any carbon, and this one was made out of BiOCl and Bi2O2Se. As the name suggests, it's a compound of bismuth, oxygen, selenium, and chlorine.
To create the new conductive material, the team found two different arrangements of the atoms in those materials that resulted in poor thermal conductivity.
They then studied the mechanisms responsible for slowing down heat in each of those arrangements, and found a way to join the two in a way that allowed them to combine the heat-slowing effects, instead of simply averaging out the difference.
In the image below you can see a visual representation of the two different atomic arrangements, represented by yellow and blue, which combine to most effectively slow down the motion of heat through the material.
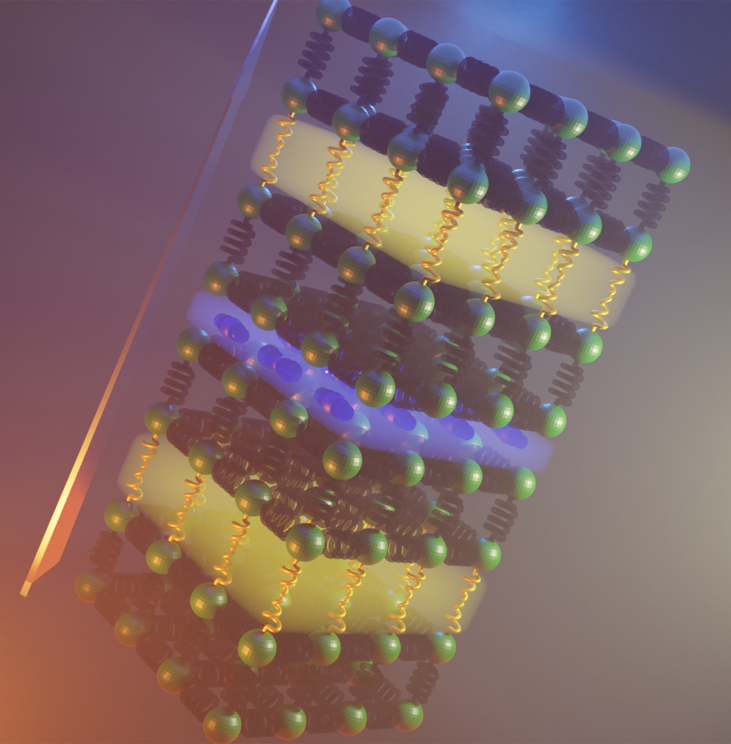 Two atomic arrangements (yellow and blue) combined to create the material.
Two atomic arrangements (yellow and blue) combined to create the material.
The result is that Bi4O4SeCl2 is a far poorer conductor of heat than either of the two arrangements are on their own, achieving room temperature heat conductivity of just 0.1 W K−1 m−1. In other words, the material is a sum greater than its parts.
It's important to note that this study only looked at thermal conductivity of the new material, and no other effects such as electrical conductivity or magnetism. So it's not yet clear whether this material could be used in real-world applications, such as computing or in the electrical grid.
But now that we know how to layer atoms in this complicated way, it opens up a lot of potential for new materials that take these thermal conductivity properties and combine them with other desirable traits to enhance thermoelectric performance or open up superconductivity.
"This potential for multiple property optimization illustrates how synergy between modular units with compatible bonding can enable chemical generation and control of function," the researchers write.
The research has been published in Science.

