Forget what you've learned - scientists just created a stable helium compound
If you remember your high school chemistry, you'll know that helium is a bit of an oddball. This noble gas is the least reactive element on the periodic table, and thanks to its full outer shell, conventional wisdom states that helium cannot interact with other atoms to create stable compounds.
While other noble gas elements have shown signs of forming compounds under extreme pressure, helium has remained firmly exclusive - until now. Scientists report creating what appears to be a stable helium-sodium compound, and it challenges some of the most basic assumptions of modern chemistry.
"Chemistry changes when you apply high pressure, and this can be achieved inside our Earth and on different planets like Saturn," one of the team, Ivan Popov from Utah State University, told Ryan F. Mandelbaum at Gizmodo.
"But this is a book changer."
If you need a bit of a refresher on your chemistry facts, helium is the second most abundant element in the Universe, and belongs to a six-member group of elements called the noble gases - so-called because of an apparent 'aloofness' that prevents them from easily forming compounds with other elements.
Since earning their 'noble' reputation, some of these gases have shown signs of reactiveness under extreme conditions - you can actually split the noble gases up into two groups, with krypton, xenon, and radon considered to be relatively reactive, and argon, neon, and helium considered to be very unreactive.
Researchers have found ways to pair up helium with other elements in the past, but until now, the result has always been fleeting.
One of the most common examples of helium interacting with other elements refers to van der Waals forces - attractive or repulsive forces that don't require conventional covalent or ionic bonds to form.
It's known that very weak van der Waals forces exist between helium and other atoms, and at extremely low temperatures, helium can form van der Waals molecules - very weakly bound clusters of atoms or molecules - but they cannot be sustained for long.
Helium's staunch stability is due to its closed-shell electronic configuration - its outer shell is complete, which means there's no room for it to bond with other atoms by sharing electrons.
But that's assuming the conditions are consistent with what we experience on Earth's surface.
Being one of the most abundant elements in the Universe, responsible for forming stars and gas giant planets, helium could play by very different rules out in space and deep within our planet, and researchers have just found the first evidence yet of that weird behaviour.
"[E]xtremely high pressure, like that found at Earth's core or giant neighbours, completely alters helium's chemistry," one of the team, Alex Boldyrev from Utah State, told Mary-Ann Muffoletto at Phys.org.
The researchers used a 'crystal structure-predicting' computer model to predict that under extreme pressures, a stable helium-sodium compound could form.
They then physically created the never-before-seen compound, Na2He, in a diamond anvil cell experiment, which allowed them to subject helium and sodium atoms to pressures of around 1.1 million times Earth's atmospheric pressure.
"These findings were so unexpected, Boldyrev says, that he and colleagues struggled for more than two years to convince science reviewers and editors to publish their results," says Muffoletto.
Based on these results, the team predicts that sodium will easily bond with helium gas to form a stable Na2He compound under pressures up to 10 million times higher than the level they achieved it at.
And, curiously enough, the compound appears to form without any chemical bonds to hold it together.
"The compound that we discovered is very peculiar: helium atoms do not actually form any chemical bonds, yet their presence fundamentally changes chemical interactions between sodium atoms, forces electrons to localise inside cubic voids of the structure, and makes this material insulating," one of the researchers, Xiao Dong from Nankai University in China, said in a statement.
Here's the crystal structure of Na2He - a solid formation of alternating sodium (purple) and helium (green) atoms, with electrons (red) shared in the voids between them:
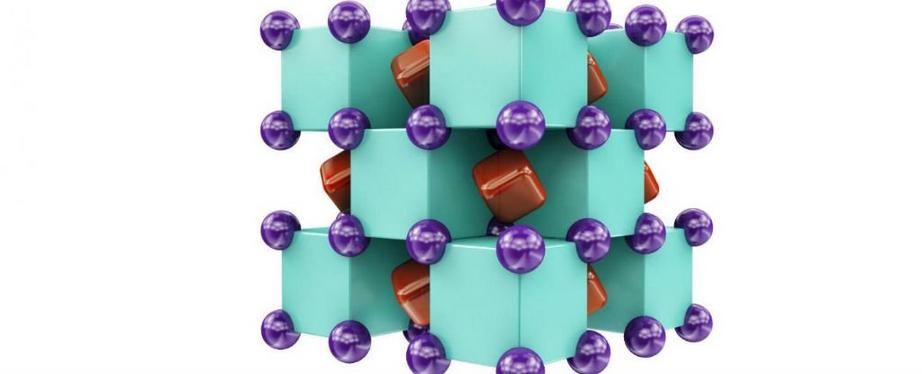
Artem R. Oganov
"It's not a real bond," like ionic and covalent bonds, Popov told Gizmodo. "But [the helium] does stabilise the structure. If you take those helium atoms away, the structure will not be stable."
Here are a couple more representations, with pink sodium and white helium on the left; and sodium and helium cubes in grey and electrons in red on the right:
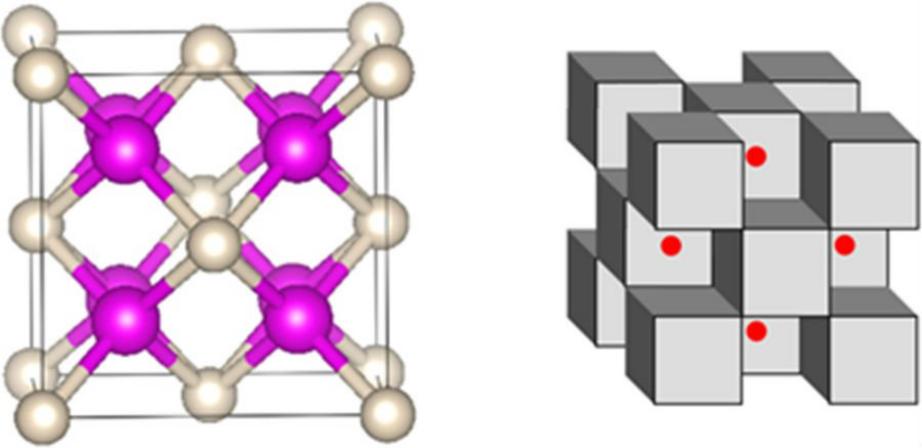
Xiao Dong et. al.
Chemists have made a number of these 'rule-breaking' discoveries recently, with separate teams creating the world's first sample of metallic hydrogen, and a carbon molecule with six - not four - bonds last month.
But because they defy conventional wisdom, these kinds of discoveries are often met with a lot of skepticism prior to independent replication of the results.
To this study's credit, the results are looking solid so far, so we can expect to see some really interesting results from follow-up experiments.
"This is much sounder science," physicist Henry Rzepa from Imperial College, London, who was not involved in the study, told Gizmodo when comparing the helium bonds with metallic hydrogen discovery.
"This helium compound is a breakthrough."
More experiments will need to be done to know for sure what's going on here, but 2017 is already looking like the year where many of our old chemistry assumptions are being put to the test, and we can't wait to see what happens next.
The results have been published in Nature Chemistry.
