Virotherapy: a new era in cancer treatment?
Editor’s Note: This article first appeared in the April 2016 edition of TTAC’s Heroes Against Cancer member newsletter.
The year 2015 was a busy one for me, as I spent many months traveling the world in search of everything there is to know about cancer – and more importantly, the many ways in which this prominent disease is being successfully treated throughout the world.
During my travels, I came across a revolutionary approach to cancer treatment that, in my humble opinion, just might be one of the most promising cancer therapeutic yet to come along in the 21st century.
It’s known as Rigvir® virotherapy, and an astute team of scientists, researchers, and doctors in the European country of Latvia are successfully using it right now to treat and cure many different types of cancer. Among the many reasons why I believe Rigvir® is so promising is the fact that it’s completely safe and 100 percent natural,derived from beneficial viruses that live naturally inside the human body. Rigvir® is also highly effective at treating many forms of cancer, which I’ll expound upon later.

Similar in function to the probiotic bacteria that populate the small intestine, the viruses from which Rigvir® is derived are recognized as beneficial pathogens that support the body’s innate immune system. These valuable viruses are both oncotropic and oncolytic, meaning they selectivelytarget and destroy cancer cells. They do this without harming healthy cells, which can’t be said about conventional therapeutics such as chemotherapy and radiation.
I found the premise behind Rigvir® virotherapy as an efficacious cancer treatment so fascinating that I decided to conduct a series of interviews with the incredible minds behind its development. I also took the time to listen to a number of powerful life stories from people whose lives have been forever changed by this amazing treatment, which are nothing short of inspirational.
Discover the Amazing Healing Power of Rigvir®
The idea that viruses can act as helpful scavengers to root out infection and disease isn’t necessarily new. Saint Peregrine, the Roman Catholic patron saint of cancer patients who lived from 1265-1345, is said to have overcome an ulcerative growth on his leg as a result of a mystery virus that lived inside his body. Fast-forward about 600 years and the word “virotherapy” made its first appearance in the PubMed database around 1960.
It was around this time that emerging research began to toy with the idea that viruses could potentially be used as virotherapeutics to alter the way cancer grows and spreads. This hypothesis turned out to be correct – viruses directly affect the growth and spread of cancer cells. And now, thanks to the blood, sweat, and tears of scientific progressives in Latvia, we finally have access to the world’s first science-based virotherapeutic in the form of Rigvir.®
Rigvir® has its origins in the Latvian capital of Riga, where scientists there began looking at it as a possible virotherapeutic as far back as the late 1960s. A shorthand derivative of “Riga Virus,” Rigvir® is based on a virus known as Echo-7 that was first extracted from the gut microflora of healthy children as part of early medical testing for the polio vaccine.
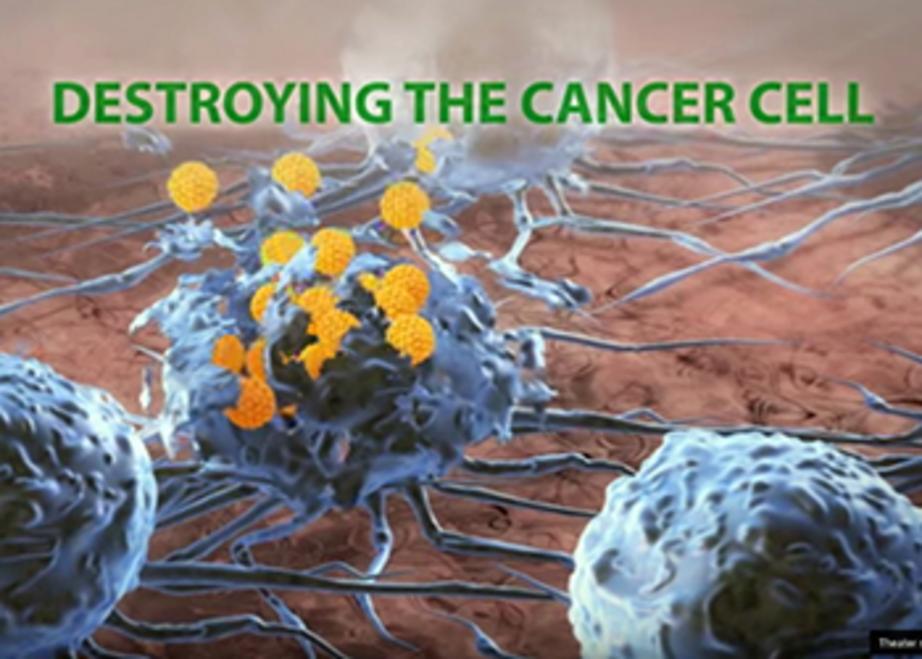
The Rigvir® virus enters a cancer cell and reproduces until the cell is destroyed
After this testing was completed, scientists began to take a closer look at the many isolated viral samples they had on hand, discovering in the process that Rigvir® possesses unique anti-cancer potential. They learned that Rigvir® is able to gain unique entry into cancer cells where it perpetually reproduces until those cells are eventually destroyed.
When Rigvir® is introduced into a cancer patient’s body via intramuscular injection, it very rapidly activates the body’s natural immune response, as well as the immune cells found inside the lymph nodes. It then actively and aggressively seeks out cancer cells, attaching to their surfaces for penetration, replication, and ultimate destruction.
Clinical testing has shown that Rigvir® acts quickly and powerfully in eradicating cancer cells throughout a patient’s body, showing near-immediate effects. Although it’s only been approved in two countries (Latvia and Georgia), for use in the treatment of melanoma, Rigvir® has also shown incredible success in the treatment of:
- Colorectal cancer
- Kidney cancer
- Bladder cancer
- Prostate cancer
- Stomach cancer
- Pancreatic cancer
- Uterine cancer
- Lung cancer
- Several types of sarcoma cancer

The Rigvir® virus is injected into the muscle. From there it activates the body’s immune response, including immune cells in the lymph nodes
What sets Rigvir® apart from many of the other cancer therapies out there is its purity. The only thing in a vial of Rigvir®is the virus and purified saltwater – that’s it! None of the preservatives, heavy metals, or adjuvants commonly found in vaccines and pharmaceuticals are present, which makes Rigvir®exceptionally safe and virtually side effect-free.
“[Rigvir®] contains the virus and almost nothing else except for normal saline for injection,” says Professor Ivars Kalvins, PhD, a scientist, inventor, and Director of the Latvian Institute of Organic Synthesis, which produces Rigvir® treatments for patients.
“This means that by administration of this (treatment), the body is not exposed to anything else, no additives, no toxic substances, only the live virus that upon entry into the body fluids starts searching for tumour cells – tumour cells that the virus eventually infects and kills.”
 Dr. Ivar Kalvins (left) helped pioneer the development of Rigvir®
Dr. Ivar Kalvins (left) helped pioneer the development of Rigvir®
Dr. Ivars Kalvins, who helped pioneer the development of Rigvir,® was one of three finalists for the European Medicine Award. Dr. Kalvins is proud of Rigvir’s® successful track record as seen over the past 12 years since it first gained commercial approval.
For my docu-series, The Truth About Cancer: A Global Quest, I got a chance to speak with Dr. Kalvins about the nature of Rigvir® and how it works. He told me that, in addition to its immunomodulating efficacy, Rigvir® is characterized as being selectively toxic, meaning it doesn’t harm healthy cells.
 Rigvir® has demonstrated numerous benefits without the harmful side effects of many coventional cancer treatments
Rigvir® has demonstrated numerous benefits without the harmful side effects of many coventional cancer treatments
Most other drugs, he told me, “are also killing healthy cells, not only the cancer cells. The only way to overcome this is to use living species like viruses. If you can find the virus [that specializes at] finding cancer cells and penetrating into these cells using the machinery, the factory, of these cells to reproduce themselves, this is exhausting the resources of (cancer) cells, and cells are dying out. This is the new approach. There is only one medicine in the world on the market that uses live virus – Rigvir.®”
Rigvir® truly is a one-of-a-kind cancer treatment, and the first ever virotherapeutic in the world to gain commercial approval. And I’m excited to say that it’s helping people from every corner of the globe overcome cancer, including late-stage cancers that the medical industry says are “incurable.”
Real-Life Testimonies from Patients Cured of Cancer with Rigvir®
One sweet Ukrainian lady I interviewed on my trip to Latvia is Khrystyna Yakonvenko. She told me her amazing story of overcoming stage IV melanoma with Rivgir.® Stage IV melanoma, as you may already know, is considered by the conventional medical system to be incurable due to the fact that cancer cells have already spread from the lymph nodes to vital organs and soft tissue.
“It all started in the end of 2012,” Khrystyna explained. “When I contacted my doctor, he diagnosed melanoma of the fourth stage, with metastasis of the liver. They prescribed the palliative chemotherapy, and … I completely trusted our Ukrainian doctors and the methods they were using, and I trusted this palliative chemotherapy – I simply didn’t realize the effect of this diagnosis completely and entirely in that moment.”
When I asked Khrystyna how long she was expected to live, “maximum of about six months,” was her response, according to her doctors’ assessment. But Khrystyna didn’t let this diagnosis get the best of her.
 Khrystyna Yakonvenko wipes away a tear as she tells how she almost died from Stage 4 melanoma prior to receiving Rigvir® therapy
Khrystyna Yakonvenko wipes away a tear as she tells how she almost died from Stage 4 melanoma prior to receiving Rigvir® therapy
“I was not feeling afraid; I was not falling into panic,” she told me, as tears began to form. “Simply, I understood that I had no right to live it, and I had to fight. When I first came to the Rigvir® therapy center, the doctors didn’t say that, yes, we will do it – they said, we will try, because the stage is late. And I think that sometimes on the earlier stages, on the initial stages, people who have this very scary diagnosis, they sometimes by themselves, they lose hope, they stop fighting, and they simply leave it.
But sometimes there are people who, even at the late stages, they continue to fight, they continue to find the way out of the situation, and in this case, the disease is simply just over,” Khrystyna explained, noting that her stage IV melanoma is now completely gone.
Zoya Sokolova from Russia had a similar story to tell, except hers started off much differently. After being diagnosed with Stage 3 sarcoma, Zoya decided to go the conventional treatment route, only to end up on the verge of death! Fortunately for Zoya, her family intervened and took her to get Rigvir® Virotherapy before it was too late.
The diagnosis was very sudden for me. It was after a very strong stress, and then after a month and a half I was diagnosed with a third-stage cancer. After the surgery, I had my chemotherapy, then I signed (up for) another six of chemotherapy, and a full course of radiotherapy.
My condition allowed only for chemotherapy to be handled, and after the fourth, I wasn’t able to stand up from the bed. I became a bed patient. Before these courses of chemotherapy, I was told about the (Rigvir®) center, about this treatment, but I believed in our doctors and their treatment methods, so I decided to follow that path.
It seemed that they wanted for me to be treated, but they couldn’t give a warranty. They couldn’t say for sure that [they] would treat this disease. And that moment when I couldn’t stand up from the bed on my own completely, I was so weak, and my relatives they decided to take a van to make a bed for me, and simply drive me to Riga to the center.
Before coming to Riga, I got a blood test and a complete observation for the doctors here to have the full picture of my condition. When the doctor saw my blood test, she was really astonished because the other blood test was lower than for a live person. She was astonished how I managed to get here staying alive.
The doctor said exactly, ‘I’m not asking how you got here, I’m asking how are you still alive with this blood test?’ From my feelings at that moment, I realized that I would not survive, and I felt how my body is failing from day to day. The doctors recommended that I take certain measures, to take certain indications… before even taking Rigvir,® to boost my immune system, to reinvigorate and repair my immune system and my body so I could be ready for Rigvir.® And after a couple of weeks, I started to take Rigvir.®
It was really my family who made the decision because, as for me, I was so weak, I was so done, that I simply took this and I was ready to say goodbye to the life, [but] my family made the decision. I simply accepted their decision and I thought, what shall be shall be, [so] I followed them.
After following the indication of the doctors for boosting my body and my immune system [for] two weeks, I was able to stand up from the bed and walk around the house and move, so I was feeling better. After a month of already taking Rigvir® virotherapy, my condition changed entirely. I was now even able to walk around, stand up from the bed, I was even able to drive myself to Riga to receive the therapy.
 Zoya Sokolova started chemotherapy treatments with a group of women who all rejected Rigvir® treatment as an option. Zoya is the only cancer survivor from the group
Zoya Sokolova started chemotherapy treatments with a group of women who all rejected Rigvir® treatment as an option. Zoya is the only cancer survivor from the group
Zoya’s story is a tearjerker, but she was the fortunate one. The other women with whom she had been receiving treatment for similar cancers while still in Russia weren’t so fortunate, as all of them rejected the Rigvir® treatment in favor of chemotherapy and radiation. And all of them paid for this decision with their lives.
“When I had my surgeries, I had them with the same women in the same room, so we were following the same path, and met on the same days for chemotherapy. And when I started Rigvir,® even though I had only four (regimens of) chemotherapy, not the full course, they continued this course, and as I was getting better with Rigvir,®they on chemotherapy were starting to feel worse and worse,” recounted Zoya.
When I started to receive Rigvir® therapy, very quickly I became a very healthy person. I started to travel, I started to have a lot of energy and I called them and I recommended that they start this treatment. But for some reasons they refused, and now they’re all gone.
It’s a pity that a lot of great people are gone now. But I’m happy, I’m healthy, and I can’t say that at the beginning I didn’t trust this method, simply I didn’t know and I was so weak. But now, currently, I don’t have anymore disabilities, I’m a completely healthy person and I’m so thankful to the people who have helped me here.
I can’t even begin to tell you how much joy was brought to my heart knowing that these two lovely women are still with us today and living vibrant lives thanks to Rigvir®Virotherapy. The same can be said for many other patients like them who I personally met in Latvia, including:
- Karlis Venskus, who was cured of Stage 4 stomach cancer with Rigvir®
- Egidijus Kazlauskis, who was cured of Stage 3 melanoma with Rigvir®
- Gunars Strazdinsh, who was cured of Stage 3 small cell lung cancer with Rigvir®
- Ruslan Isayev, who was cured of Stage 3 skin melanoma with Rigvir®
- Svetlana Sheferova, who was cured of Stage 3 melanoma with Rigvir®
FDA Approves GMO Knockoff of Rigvir® Cancer Treatment
You’re probably asking yourself right about now – how can I access Rigvir® here in the United States for myself or a loved one? The unfortunate answer is that Rigvir® isn’t approved for use in the U.S., and is only available in Latvia, Mexico, the Bahamas, Germany, and the country of Georgia.
The reason for this is a systemic bias within the U.S. Food and Drug Administration (FDA) that prevents life-saving cancer treatments that actually work from ever being commercially approved. I cover this conspiracy in considerably more depth in my docu-series, but suffice it to say that the cancer industry has a stranglehold on the ways in which cancer can be legally treated here in the States.
To make matters worse, the FDA did recently approve another form of oncolytic virotherapy known as “Imlygic”™ (talimogene laherparepvec) that, like Rigvir,® is the first of its kind to be approved for use in the treatment of melanoma. But unlike Rigvir,®Imlygic™ is genetically-modified (GMO) and non-selectively toxic, meaning it harms both benign and malignant cells.
Even worse is the fact that Imlygic™ isn’t even all that effective, nor does it root out advanced-stage melanomas that have already spread to vital organs and other soft tissue. Imlygic,™ which is made of GMO herpes virus, can also infect patients with herpes, as admitted by the FDA in a recent press announcement.
A mere 16.3 percent of melanoma patients treated with Imlygic™ in a multi-center clinical trial saw any improvements at all from the drug’s use, according to the FDA. And the drug did not show benefits among patients whose melanomas had already spread to the brain, bone, liver, lungs, or other internal organs.
 According to the Imlygic website, “IMLYGIC™ has not been shown to improve overall survival or have an effect on visceral metastases” (meaning cancer of internal organs)
According to the Imlygic website, “IMLYGIC™ has not been shown to improve overall survival or have an effect on visceral metastases” (meaning cancer of internal organs)
Imlygic™ is also loaded with various chemical additives like disodium hydrogen phosphate dihydrate (pH balancer) and sorbitol (sugar alcohol) that have a questionable safety profile when injected directly into muscle tissue. Rigvir,® as I mentioned earlier, is made up of just the natural virus and saltwater.
Imlygic™ pales in comparison to Rigvir® on every level from its safety to its effectiveness, not to mention the fact that Imlygic™ is made from a recombinant (genetically engineered) herpes virus that exists nowhere in nature, while Rigvir® is made from a naturally-occurring virus that is simply an extension of the body’s own native immune system.
We can’t keep shunning effective treatments like Rigvir® in favor of questionable ones like Imlygic,™ simply because drug companies (in this case Amgen), have enormous amounts of cash to shell out to the FDA in exchange for a regulatory stamp of approval. The system is broken, and tens of thousands, and even millions, of people are needlessly dying from preventable diseases as a result.
It is my sincere hope that someday our country will recognize other forms of medicine besides just pharmaceuticals and surgery, and that cancer patients who wish to undergo treatments that fall outside the norm will be able to do so, within our own country, legally, and with full support of health insurance providers.
This article first appeared in the April 2016 edition of TTAC’s Heroes Against Cancer newsletter. Each month in Heroes Against Cancer we share the best ways you can use to get and stay healthy – including delicious recipes and the best in supplements, herbs and spices. Find out more about our member newsletter here.
For full references please use source link below.