Pfizer Docs: 800+ people never finished jab trial due to injury or death
Pfizer Documents reveal at least 800 people never finished the COVID Vaccine Trial due to Death, Injury or Withdrawn Consent
By The Exposé on June 15, 2022
One of the confidential Pfizer documents that the U.S. Food and Drug Administration (FDA) has been forced to publish by court order reveals that approximately 800 people never completed the phase 1 Pfizer Covid-19 vaccine clinical trial in the USA due to either losing their life, suffering a serious adverse event or suddenly withdrawing their consent.
Let’s not lose touch…Your Government and Big Tech are actively trying to censor the information reported by The Exposé to serve their own needs. Subscribe now to make sure you receive the latest uncensored news in your inbox…
Follow The Exposé’s Official Channel on Telegram here
Join the conversation in our Telegram Discussion Group here
The US Food and Drug Administration (FDA) attempted to delay the release of Pfizer’s COVID-19 vaccine safety data for 75 years despite approving the injection after only 108 days of safety review on December 11th, 2020.
But in early January 2022, Federal Judge Mark Pittman ordered them to release 55,000 pages per month. They released 12,000 pages by the end of January.
Since then, PHMPT has posted all of the documents on its website. The latest drop happened on 1st June 2022.
One of the documents contained in the latest data dump is ‘125742_S1_M5_5351_c4591001 fa interim discontinued patients.pdf’.
The document provides a 112-page list of subjects who withdrew from the phase 1 clinical trial of the Pfizer Covid-19 injection, and provides a vague description as to why.
The first 14 pages list 102 subjects who withdrew from the study. This equates to an average of 7.2 subjects per page. So based on a further 93 pages detailing withdrawn subjects this equates to approximately 780 people who withdrew from the first phase of the clinical trial alone. The actual number could be slightly more or slightly less.
Many of the subjects mysteriously revoked consent to continue in the trial due to reasons such as re-reading the consent form and deciding it is not what they had originally agreed to.

Whilst others withdrew their consent to continue in the study following receipt of dose 1 for unexplained reasons, meaning they did not want to receive the second dose.

But unfortunately, there are several who withdrew their consent to continue the trial due to suffering serious adverse events. Page 110 of the document lists one person who suffered a pulmonary embolism, which is a blood vessel in the lungs blocked by a blood clot. The condition can be life-threatening if not treated quickly.

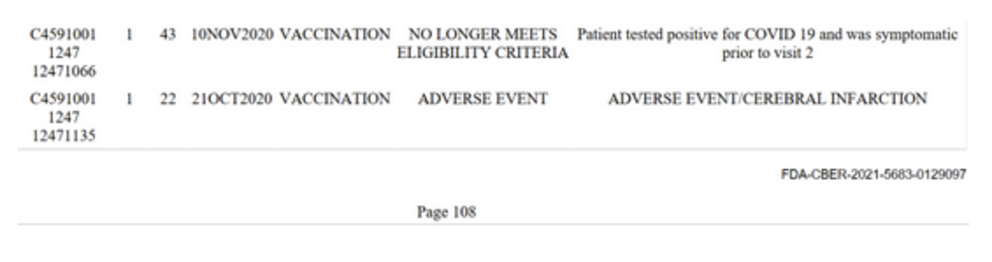
Page 108 of the document lists one person who suffered a cerebral infarction. The condition is also known as an ischemic stroke and occurs as a result of disrupted blood flow to the brain due to problems with the blood vessels that supply it. A lack of adequate blood supply to brain cells deprives them of oxygen and vital nutrients which can cause parts of the brain to die off.
Page 102 of the document lists a person who suffered a transient ischaemic attack. The condition is also known as a mini-stroke and is again caused by disruption in the blood supply to the brain. Unfortunately, due to suffering the mini-stroke, this person also fell and fractured their ankle at the same time.

Page 100 of the document lists a person who withdrew consent to continue in the trial due to losing their hearing and going completely deaf in one ear. The page also lists another person who suffered ‘syncope’ which is a temporary loss of consciousness usually related to insufficient blood flow to the brain.

Page 94 of the document lists a person who withdrew consent due to suffering tachycardia. The condition refers to a heart rate that is too fast and can be caused by poor blood supply to the heart muscle.

Page 44 of the document lists a person who withdrew consent due to suffering paraparesis. The condition refers to partial paralysis in both legs due to disrupted nerve signals from the brain to the muscles.

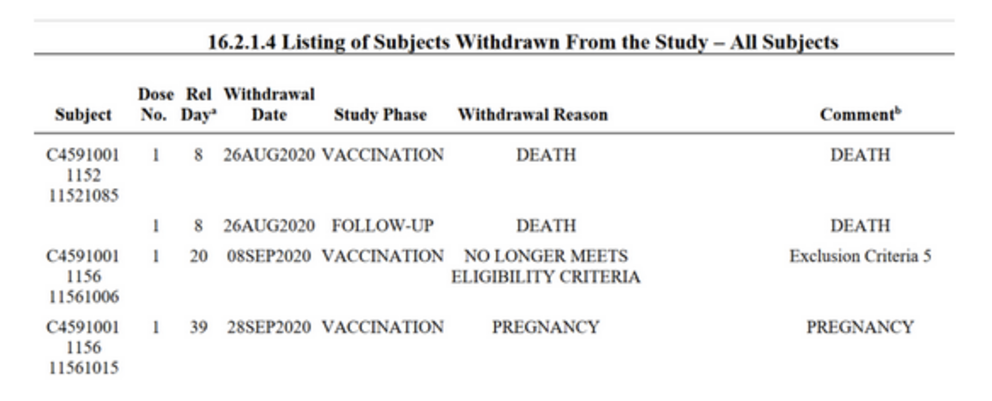
Sadly, the document also lists several people who were no longer able to take part in the phase 1 clinical trial due to losing their lives. Several deaths can be viewed throughout the document including, but not limited to pages 106/107, 101, 80 and 47.



We know why those who sadly died or were injured didn’t complete the trial. But why did hundreds more refuse to continue the phase 1 clinical trial of the Pfizer Covid-19 vaccine in the USA after being eager to participate originally?
And why were the FDA desperate to hide this document among many others for at least 75 years?
Subscribe now to make sure you receive the latest uncensored news in your inbox…
Email Address
The Expose is now censored by
Google, Facebook, Twitter & PayPal.
So we need your help to ensure
We can continue to bring you the
facts the mainstream refuse to…
We’re not funded by the Government
to publish lies & propaganda on their
behalf like the mainstream media.
Instead, we rely solely on our support. So
please support us in our efforts to bring you
honest, reliable, investigative journalism
today. It’s secure, quick and easy…
Just choose your preferred method
to show your support below support
Send Bitcoin
The Expose Bitcoin Wallet Address –
3KpsgfuEX6v7w83aVN4b1dfCZTzas7Kt74
Send Monero