Pfizer CEO made ‘misleading’ comments about COVID shots for kids, UK regulator finds
Pfizer CEO Albert Bourla, Ph.D., made “misleading” and “unqualified” comments promoting the use of COVID-19 mRNA vaccines for young children during an interview on the BBC, a U.K. regulatory agency found.
This article was originally published by The Defender — Children’s Health Defense’s News & Views Website.
Albert Bourla. Photo credit: World Economic Forum/flickr.
Miss a day, miss a lot. Subscribe to The Defender's Top News of the Day. It's free.
Pfizer CEO Albert Bourla, Ph.D., made “misleading” and “unqualified” comments promoting the use of COVID-19 mRNA vaccines for young children during an interview on the BBC, a U.K. regulatory agency found.
The Prescription Medicines Code of Practice Authority (PMCPA), an independent, self-regulatory body established by the Association of the British Pharmaceutical Industry (ABPI), found Bourla breached several rules in its Code of Practice for advertising.
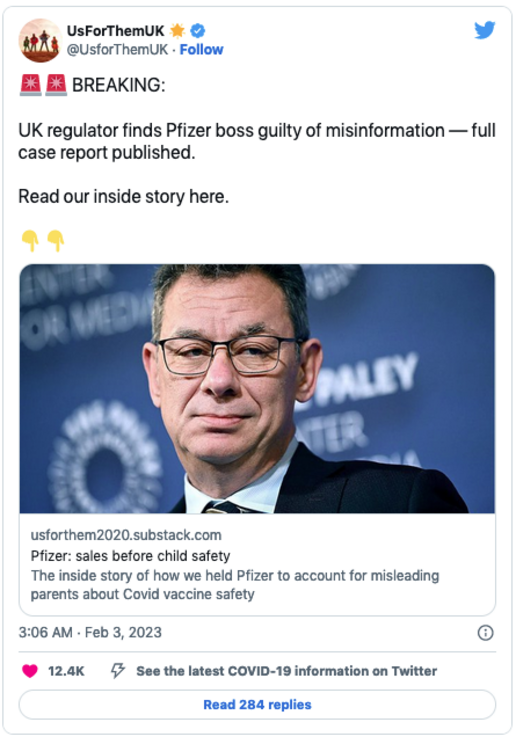
U.K. pharmaceutical industry watchdog UsForThem filed the complaint with the PMCPA. In a Substack post after the ruling, UsForThem accused the BBC’s medical editor, Fergus Walsh, of conducting the interview “as a friendly fireside chat,” giving Bourla “a free pass promotional opportunity that money cannot buy” by allowing him to promote vaccine uptake, particularly among young children for whom the vaccine had not even been authorized.
As the U.K.’s national public service broadcaster, the BBC is meant to follow strict guidelines regarding commercial advertising or product placement, which UsForThem said the Bourla interview failed to follow.
The BBC published the interview with Bourla in December 2021 on its website, its news app and in the “BBC News at One” program, as a video interview and an accompanying article, “Pfizer boss: Annual Covid jabs for years to come.”
The interview aired two days after the U.K. government announced it had agreed to purchase 54 million more doses of the mRNA vaccines from Pfizer-BioNTech and 60 million more from Moderna.
The PMCPA can fine Bourla only for administrative costs. It does not have the authority to impose other penalties.
The BBC is the founding member of the Trusted News Initiative (TNI). Children’s Health Defense last month sued the BBC and three other TNI members, alleging they partnered with several Big Tech firms to “collectively censor online news,” including stories about COVID-19 that were not aligned with official narratives regarding those issues.
Bourla: Immunizing young children ‘would be a very good idea’
In the BBC interview, Bourla said it was up to the regulatory agencies to determine whether to approve and distribute vaccines to children under 11, but he thought that “immunising that age group in the UK and Europe would be a very good idea,” according to the PMCPA case report published last week.
At the time, no COVID-19 vaccines had been approved by the U.K.’s Medicines and Health products Regulatory Agency (MHRA) for children under 12, so the panel found Bourla’s comments were in breach of code.
Citing possible disruptions in schooling and the potential for long COVID, Bourla also said, “So, there was no doubt in my mind that the benefits completely were in favour of doing it [vaccinating children against COVID-19].”
He added, “I believe it’s a good idea.”
The panel found these strong opinion statements could lead the public to infer there was no need to be concerned with potential side effects or that the benefits of vaccination outweigh the risks, which had not been determined by the health authorities.
On Dec. 11, 2021, UsForThem filed its complaint with the PMCPA citing the promotional nature of the BBC’s reports and Bourla’s failure to comply with U.K. rules governing the promotion of medicines.
After the PMCPA ruled Bourla’s statements breached a number of rules in the ABPI’s code of practice, Pfizer appealed, including that his statements were of a “strong unqualified nature.”
The regulator also said the statements implied there was “no need to be concerned about potential side-effects of vaccination in healthy children aged 5-11” and that the implication was “misleading and incapable of substantiation.”
The appeal board upheld five counts of breaches of three ABPI codes that require information and claims “to be accurate, balanced, capable of substantiation, not raising unfounded hopes of successful treatment, and not be misleading with respect to the safety of the product,” The Epoch Times reported.
The PMCPA posted its final ruling on Jan. 27, more than a year after the initial complaint was filed.
During that time — in February 2022 — the U.K.’s Joint Committee on Vaccination and Immunisation ruled children ages 5-11 could be offered the vaccine, but the committee said the recommendation was “non-urgent.”
UsForThem celebrated on Twitter:
Neither Pfizer nor Bourla commented publicly on the ruling.
The Epoch Times reported that in a November 2022 statement on the case, a spokesman for Pfizer said the company was “committed to the highest levels of integrity in any interaction with the public.”
As of Feb. 12, the U.K. will no longer recommend COVID-19 boosters for healthy people under age 50, and will discontinue free distribution of the primary two-shot series, The Defender reported.
Subscribe to The Defender - It’s Free!
Sign up for free news and updates from Robert F. Kennedy, Jr. and the Children’s Health Defense. CHD is planning many strategies, including legal, in an effort to defend the health of our children and obtain justice for those already injured. Your support is essential to CHD’s successful mission.
This article was originally published by The Defender — Children’s Health Defense’s News & Views Website under Creative Commons license CC BY-NC-ND 4.0. Please consider subscribing to The Defender or donating to Children’s Health Defense.