FDA - captured and corrupt
Story at-a-glance
- While the U.S. Food and Drug Administration itself does not accept corporate money, it does receive money funneled via a nonprofit foundation, which receives money from other nonprofits funded by private interests
- The Reagan-Udall Foundation is a nonprofit foundation created by Congress in 2007 to support scientific research that is of interest to the FDA. It accepts grants from government, individual donors and other nonprofits — even when those nonprofits are created and funded by industry
- The Reagan-Udall Foundation has received large donations from the Bill & Melinda Gates Foundation
- Ellen Sigal, who currently chairs the Reagan-Udall Foundation’s board of directors, is also vice president of the Cancer Moonshot program, funded by the Gates Foundation, and she’s on the board of the Parker Institute, which is partnered with Inovio, a Gates-funded company that is currently working on a COVID-19 vaccine
- According to the rules, no more than four of the 14-member board of the Reagan-Udall Foundation are supposed to be representatives of FDA-regulated industries, yet in 2017, nine of the then 13-member board had financial ties to industry at the time of their appointment
If you’re like most people, you probably assume that the U.S. Food and Drug Administration is funded by the U.S. government and therefore isn’t catering to private industries.
The agency itself certainly tries to present itself as independent from the industries it regulates but, in reality, legal loopholes have led to the FDA receiving money from, and being captured and corrupted by, private interests.
While the FDA itself does not accept corporate money, it does receive money funneled via a nonprofit foundation, which in turn receives money from other nonprofits funded by private interests. It’s really all a façade because the end result is the same. Those donating the money ultimately end up with the ability to pull strings, when needed.
The Reagan-Udall Foundation
As explained by NPR1 back in 2012, the Reagan-Udall Foundation is a nonprofit foundation created by Congress in 2007 to support scientific research that is of interest to the FDA. According to NPR:2
“The idea was that this foundation could do things the FDA can't. It would raise money from private sources, fund research in areas where the FDA lacks expertise, and organize collaborations involving industry, patient groups and academia.”
As explained in a 2008 article3 in the Journal of the National Cancer Institute, the creation of the Reagan-Udall Foundation was part of a larger plan to establish a private-public partnership to facilitate the Critical Path Initiative.
The Critical Path Initiative was part of the FDA’s attempts to streamline and modernize the drug approval process by having companies pay user fees. Part of the Reagan-Udall Foundation’s responsibilities was to set goals and priorities for the Critical Path Initiative, and then award grants to meet those goals.
Massive Loophole: Nonprofits Funded by Industry
However, critics voiced concern, saying the Reagan-Udall Foundation might allow the food and medical industries “to sway FDA decisions,” since it could raise money from private, including industry, sources. To quell some of these fears, the Reagan-Udall Foundation said it would only accept grants from government, individual donors and other nonprofits, not industry.
After a few years of scraping by on small, private donations, the foundation received a $150,000 grant from the PhRMA Foundation, another nonprofit foundation funded by drug companies. Being a nonprofit, the PhRMA Foundation fit the description of an acceptable funding source, but just how independent can it actually be when it’s founded and funded by drug companies?
As noted by consumer advocate Sidney Wolfe with Public Citizen, while the PhRMA Foundation is technically a nonprofit, “one can hardly expect that they're going to do things that are not in the interests of their funders."4
Indeed, and this influence is in addition to the influence food, drug and medical device companies already have, by way of user fees. Again, the Prescription Drug User Fee Act established an accelerated application process for new drugs. The sped-up process is funded through industry-paid fees.
This fee, however, works more like a payoff or soft bribe. When a company pays the FDA for an accelerated review, the agency no longer has an incentive to find fault with the product or demand more extensive testing.
FDA Foundation Funded by the Gates Foundation
Not surprisingly, the Reagan-Udall Foundation has received large donations from the Bill & Melinda Gates Foundation, which we now know rarely does anything that doesn’t benefit Gates’ personal bottom line and overall agenda.
As detailed in “Bill Gates — Most Dangerous Philanthropist in Modern History?” Gates has used his philanthropy to shape public policy in ways that benefit his own agenda.
A March 17, 2020, article5 in The Nation titled, “Bill Gates’ Charity Paradox,” even points out that the Gates Foundation has given $2 billion in tax-deductible charitable donations to private companies, including GlaxoSmithKline, Unilever, IBM, Vodafone, the Mastercard affiliate MasterCard Labs for Financial Inclusion,6,7 Scholastic Inc. and NBC Universal Media.8,9
Many of these so-called donations end up benefiting the Gates Foundation, as it also invests in the very same companies and industries that it donates money to. This circular economy is why Gates just keeps getting richer, the more money he gives away.
Part of this wealth growth also appears to be due to the tax breaks given for charitable donations. In short, it’s a perfect money-shuffling scheme that limits taxes while maximizing income generation.
If donating to for-profit companies sounds oddly illegal to you, you’d be right. Gates is a tax evader for doing so — he’s simply getting away with it. The nonprofit foundation is a disguise to avoid taxes while funding the research arms of for-profit organizations that his foundation is invested in, which is illegal.
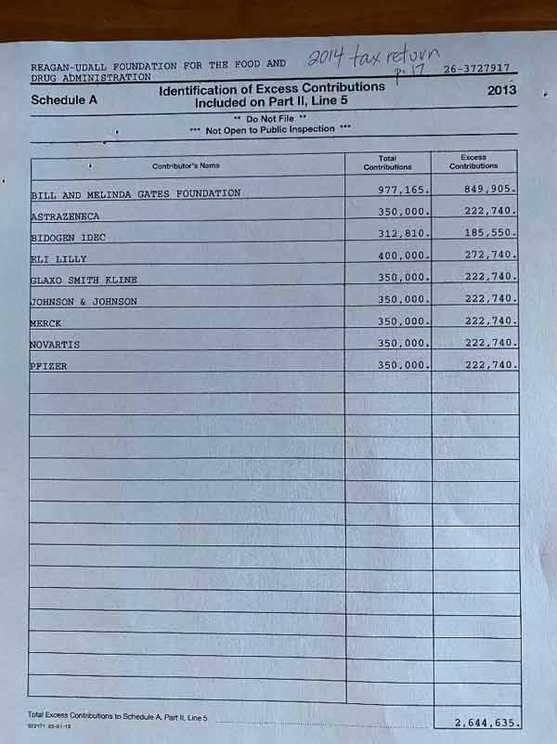
The image below shows donations received by the Reagan-Udall Foundation in 2013. Topping the list is the Gates Foundation, whose contributions for the year amounted to $977,165, followed by a string of drug companies.
Board Members With Ties to Industry
In addition to all of this financial clout, food, drug and medical device makers also have the ability to exert influence over the FDA via the members10 of the Foundation board, and this was a concern right from the get-go.
As reported in the 2008 Journal of the National Cancer Institute article,11 members of the then-newly created Reagan-Udall Foundation executive board had troubling ties to industry — and to the Gates Foundation, which years later (see above) ended up being a top financial donor. The article, written by Joel B. Finkelstein, reads, in part:12
“The Food and Drug Administration's most recent steps toward modernizing the drug approval process have renewed some old questions about the FDA's relationship with the industries it regulates.
Several public advocacy groups affiliated with physicians and researchers have voiced their concern over the appointment of certain members to a newly formed agency board. The groups have warned that some members may have conflicts of interest due to past or current roles as board members of pharmaceutical and biotechnology firms ...
The [Reagan-Udall] foundation's board of directors, appointed by the FDA commissioner, will be largely responsible for establishing by-laws, selecting an executive director to oversee day-to-day operations, and reporting to Congress on foundation activities and operations.
The federal statute stipulates that of the 14 members named to the board, four members should come from industry, three from academia, two from consumer or patient advocacy organizations, and one from the health provider community. The remaining four spots are open to anyone with relevant expertise.
The FDA has already chosen the members and is organizing the Reagan–Udall Foundation. However, some advocacy groups are concerned that several nonindustry members have strong ties to pharmaceutical and biotechnology companies, including one who is currently under investigation by the Senate Finance Committee.
Tadataka ‘Tachi’ Yamada, M.D., currently heads the Bill and Melinda Gates Foundation's global health program but until 2006 worked as head of research for the pharmaceutical company GlaxoSmithKline.
Senate investigators have uncovered evidence suggesting that, during his tenure with the company, he may have been involved in an effort to intimidate a scientist who was raising questions about the heart risks associated with the company's blockbuster diabetes drug rosiglitazone maleate (Avandia).”
While the Reagan-Udall Foundation is the nonprofit arm of the FDA, the agency does not have the authority to set conflict-of-interest policies for the foundation.13 This, of course, leaves the door wide open for conflicts of interest and allows the Foundation to become a hidden back door of sorts, for corporate influence.
Industry Dictates Level of Evidence FDA Should Use
A more recent article,14 published in 2017 in The BMJ, points out that when the Reagan-Udall Foundation is using “big data” assess drug risks and device complications, they’re using “levels of evidence recommended by industry.” The potential for manipulation should be obvious. The article, written by BMJ associate editor Jeanne Lenzer, reads, in part:15
“Big data can be used cautiously to examine real world outcomes and to improve surveillance of drug safety ... However, big data are a noisy mess, and analyses by entities with profit motives may identify spurious associations that support fast track approvals and indication creep (broadening the indications for drugs and devices).
The Reagan-Udall Foundation curates real world evidence or ‘big data’ derived from routinely collected health data from insurance claims, electronic health records, voluntary registries, and social media.
The U.S. drug and device regulator, the Food and Drug Administration, says that such data can speed up research, ‘saving time and money’ for ‘therapeutic development, outcomes research [and] safety surveillance.’
In January [2013], Robert Califf, then FDA commissioner, announced the launch of Innovation in Medical Evidence Development and Surveillance (IMEDS), a foundation project that he said would collect and analyze big data to identify ‘important safety issues.’
However, critics of the move say that big data are poor for identifying adverse events ... Financial conflicts of interest, they worry, could influence the way big data are used, including exploitation of the weaknesses inherent in observational data to win FDA approval for new uses of drugs and devices and to exonerate drugs of previously detected harms. There is evidence and precedent to support both concerns.”
Lenzer also points out that the Foundation’s board of directors still has financial ties to the drug and device makers that the FDA is supposed to regulate. She notes that while no more than four of the 14-member board should be representatives of FDA regulated industries, in 2017, nine of the then 13-member board had financial ties to industry at the time of their appointment.
The Ties That Bind
To give just one example of how conflicts of interest can have real-world implications, take the case of Ellen V. Sigal, Ph.D.16 Sigal chairs the Reagan-Udall Foundation’s board of directors.17
She’s also vice president of the Cancer Moonshot program, and it too is funded by the Gates Foundation. Sigal’s colleague at the Cancer Moonshot Program, Dr. Doug Lowy, is a co-inventor of the HPV vaccine Gardasil, and Sigal’s son, David Sigal, is married to New York State Sen. Brad Hoylman, who sponsored a bill to make Gardasil mandatory for all school children in New York.
Hoylman also supported a bill that would allow children as young as 9 to receive the HPV vaccine at school without the knowledge or consent of their parents. Gates, of course, is also a supporter of HPV vaccination and funds HPV vaccine research.
Lastly, Sigal is on the board of the Parker Institute, which is partnered with a company called Inovio. Inovio, which is funded by the Gates Foundation, is working on a COVID-19 vaccine. When you start tracing relationships, it’s amazing how often you find the Gates Foundation involved in matters relating to forced vaccinations and the destruction of legal protections.
FDA’s Lax Oversight of Clinical Research
Sad to say, it’s hard to find a government agency that hasn’t been captured by private interests. I’ve written several articles detailing the corruption at the CDC, for example, including “CDC Petitioned to Stop Lying About Pharma Funds,” “How Conflicts of Interest Have Corrupted the CDC” and “Public Health Agency Sued for Coke Collusion.”
The same can be said about the World Health Organization which, of course, is also funded by the Gates Foundation. In fact, when the U.S. withdrew its funding, Gates stepped in and became the largest funder — larger even than entire nations.
Without doubt, the FDA can be added to the list of agencies that largely serves corporate masters, hidden as they may be behind nonprofit façades. A recent investigative report18 by Science Magazine highlights the agency’s failures when it comes to overseeing clinical research, which is one of its many duties.
FDA documents obtained via Freedom of Information Act requests reveal it rarely sanctions or penalizes researchers or research companies even when grave problems — including fraud — are found.
Inspectors conduct routine visits to research trial sites and review trial records to make sure research parameters and safety protocols are followed. They also respond to complaints by whistleblowers.
However, FDA documents obtained via Freedom of Information Act (FOIA) requests reveal it rarely sanctions or penalizes researchers or research companies even when grave problems — including fraud — are found. What’s more, there’s a marked trend toward less and less adequate oversight.
Case in point: Aspen Clinical Research, run by Dr. Michael Harris, has on numerous occasions over the past decade been cited for “egregious errors” in its clinical trials, yet the FDA never followed through on its threats to fine, prosecute or disqualify Harris from conducting clinical research in the U.S. According to the report, written by Charles Piller:19
“FDA found there were serious lapses in obtaining informed consent from trial volunteers, unqualified staff made medical assessments, and Harris failed to properly report abnormal lab test results. He also did not disclose that trial participants were taking opioid, antidepressant, or antipsychotic drugs — which could have skewed results or posed safety concerns.
The agency said Aspen’s records were disorganized, contradictory, and sometimes backdated in a way that ‘begs the question of the authenticity and veracity of data collected.’ Those ‘serious, ongoing deviations’ might constitute ‘fraud, scientific misconduct,’ and ‘significant human subject protection violations,’ according to FDA documents ...
Repeat problems and a raft of new ones emerged during inspections in 2014, 2015, and 2019. Each time, in responses to FDA, Harris admitted some transgressions, strenuously disputed others, and promised to improve.
Through all that, FDA never formally sanctioned Harris or pursued other penalties. The agency never made public the alleged offenses or told trial participants they might have been put at risk. Nor did it tell companies sponsoring some of the trials that their data might have been compromised ...
Meanwhile, pharmaceutical and medical device companies continued to contract with Aspen. Since 2011, they have paid the firm millions of dollars for work on at least 65 trials, and Aspen is now recruiting people for nine new trials on Alzheimer’s disease, autism, depression, and other serious disorders.”
According to Piller, this isn’t a rare case. After reviewing some 1,600 FDA inspection and enforcement documents, Piller’s conclusion is that the “FDA’s enforcement of clinical research regulations is often light-handed, slow-moving, and secretive.”
“Clear corrections of inspector-reported dangerous or unlawful clinical trial practices were the exception, even amid signs that trial participants were harmed and that data underpinning evidence-based medicine were corrupted,” Piller writes.
“On the rare occasions when FDA formally warned researchers of findings that they had broken the law, the agency often neglected to ensure that fixes occurred ... Moreover, the agency frequently closed cases on the basis of unverified claims by those accused.”
I recommend reading Piller’s report in its entirety. It’s a sobering read that raises all sorts of questions about drug safety.
If a drug trial is riddled with errors, omissions and outright fraud and falsification of documents and data — examples of which are given in Piller’s report — and this research is then used to gain FDA approval, the chances of that drug being harmful can be considerable. Clearly, oversight without follow-up and follow-through when problems are found is about as useful as no oversight at all.
Sources and References
- 1, 2, 4 NPR April 3, 2012
- 3, 11, 12, 13 Journal of the National Cancer Institute March 5, 2008; 100(5): 296-297
- 5, 8 The Nation March 17, 2020
- 6, 9 Jacobinmag.com November 2015
- 7 PND December 8, 2014
- 10, 17 Reagan-Udall Foundation Board of Directors
- 14, 15 The BMJ 2017;358:j3275
- 16 The Fedup Democrat June 19, 2020
- 18, 19 Science Magazine October 1, 2020

