First synthetic mouse embryos - complete with beating hearts and brains - created with no sperm, eggs or womb
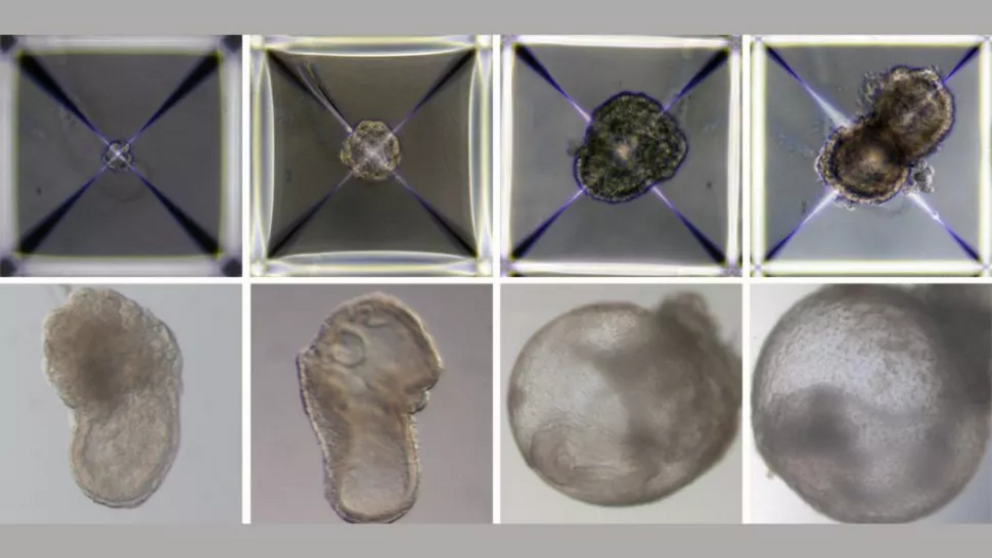
Researchers developed synthetic mouse embryos that, between day 1 (top left) and day 8 (bottom right) of growing, formed a beating heart, an emerging blood circulation, a brain, a neural tube and an intestinal tract.
This is the first time such embryos have been made.
For the first time, scientists have created mouse embryos in the lab without using any eggs or sperm and watched them grow outside the womb. To achieve this feat, the researchers used only stem cells and a spinning device filled with shiny glass vials.
The experiment is a "game changer," Alfonso Martinez Arias, a developmental biologist at Pompeu Fabra University in Barcelona who was not involved in the research, told The Washington Post (opens in new tab).
"This is an important landmark in our understanding of how embryos build themselves," he said.
The breakthrough experiment, described in a report published Monday (Aug. 1) in the journal Cell (opens in new tab), took place in a specially designed bioreactor that serves as an artificial womb for developing embryos. Within the device, embryos float in small beakers of nutrient-filled solution, and the beakers are all locked into a spinning cylinder that keeps them in constant motion. This movement simulates how blood and nutrients flow to the placenta. The device also replicates the atmospheric pressure of a mouse uterus, according to a statement (opens in new tab) from the Weizmann Institute of Science in Israel, where the research was conducted.
In a previous experiment, described in the journal Nature (opens in new tab) in 2021, the team used this bioreactor to grow natural mouse embryos, which reached day 11 of development in the device. "That really showed that mammalian embryos can grow outside the uterus — it’s not really patterning or sending signals to the embryo so much as providing nutritional support," Jacob Hanna, an embryonic stem cell biologist at the Weizmann and senior author of both studies, told STAT News (opens in new tab)
After their initial success with natural embryos, the researchers wanted to try their hand at growing lab-made embryos in the mechanical womb.
To do so, they applied a chemical treatment to mouse stem cells that "reset" them into a naive state from which they could morph into any type of cell — heart, liver, brain or otherwise. In a fraction of these naive cells, the team applied additional treatments to switch on genes required to make the placenta, and in a third group of cells they applied treatments to switch on the genes to make the yolk sac. "We gave these two groups of cells a transient push to give rise to extraembryonic tissues that sustain the developing embryo," Hanna said in the statement.
The scientists then placed these three groups of stem cells into the artificial womb to mix and mingle. The three flavors of cells soon came together to form clumps, but only about 50 out of 10,000 cellular clumps continued to develop into embryo-like structures and those that did only survived in the bioreactor for 8.5 days.
Over the course of those 8.5 days — or nearly half of a typical mouse pregnancy — the initially spherical embryos stretched out and became cylindrical, as would be expected of natural embryos, STAT News reported. The beginnings of the central nervous system began to emerge by day 6 and soon gave rise to a tiny, wrinkled brain. By day 8, the embryos had developed intestinal tracts and small, beating hearts that pushed blood stem cells through newly formed vessels.
The shape of internal structures and gene structure in the synthetic embryos differed slightly from those found in natural mouse embryos, the team noted.
In follow-up experiments, the researchers plan to study the chemical cues that push embryonic cells to become one type of tissue over another. What forces nudge certain stem cells to congregate and form the neural tube while others end up differentiating into the cells that line the intestines?
"Our next challenge is to understand how stem cells know what to do — how they self-assemble into organs and find their way to their assigned spots inside an embryo," Hanna said in the statement. "And because our system, unlike a womb, is transparent, it may prove useful for modeling birth and implantation defects of human embryos."
In addition to serving as a research model, the artificial womb could also someday serve as an incubator for cells, tissues and organs grown for transplant procedures, he said.
"This is just one step, but a very important step for us to be able to study early development," Paul Tesar, a developmental biologist at Case Western Reserve University School of Medicine who was not involved in the study, told STAT News. "We're crossing into the realm of being able to generate an embryo from scratch, and potentially a living organism. It’s been a really notable switch for the field."
Of course, such research comes with heavy ethical considerations.
"The mouse is a starting point for thinking about how one wants to approach this in humans," Alex Meissner, a stem cell biologist at the Max Planck Institute for Molecular Genetics, told The Washington Post. "It's not necessary to be alarmed or raise any panic, but … as we learn, it's important to have in parallel the discussion: How far do we want to take it?"
Originally published on Live Science.
Video can be accessed at source link below.